Hyaluronidase
History, Background & Drug Development Rationale
Hyaluronidases are a family of glycosaminoglycan-depolymerizing enzymes, that catalyze hydrolysis of
hyaluronan(HA) in the extracellular matrix(ECM) of the hypodermis.

Hyaluronan(HA) is a mega-Dalton polysaccharide with molecular weight of up to 8000 kDa and its alternating disaccharide units support the extracellular matrix to resist against the compressive forces.
It is extremely hydrophilic, forms a gel like substance with surrounding water molecule, and so establishes barrier to the bulk fluid flow as cellular cement in the subcutaneous connective tissues.
Hyaluronidase increases the hydraulic activity and opens the microscopic channels between the individual collagen fibers in the interstitial connective tissues by breaking down hyaluronan to disaccharide.
As a direct result, drugs co-administered with hyaluronidase up to 200 nm in diameter are allowed and facilitated to traverse the capillaries or lymphatics before entering the systemic circulation.
Antibodies with approx. 10nm in diameter pass through the channels when co-administered with hyaluronidase and absorbed consistently and predictably in the hypodermis to reach their intended targets.
The activity of hyaluronidase as spreading factor and dispersion enhancer were initially discovered from experiments using extracts of rabbit testes in the late 1920s (Duran-Reynals,1928,1933)
Among 6 types of hyaluronidases, HYAL-1,2,3,4 and PH20(SPAM-1, Sperm specific membrane protein, GPI anchored) and HYALP1(pseudogene1), PH20 is the only hyaluronidase developed as drug because it is active at physiological pH 7, while other hyaluronidases are only active at acidic pH 3-4.
Animal testes-derived PH20 hyaluronidases (bovine and ovine) have been used clinically as spreading factor and dispersion agent since 1940s.
Hyaluronidase catalyzes hydrolysis of Hyaluronan between beta 1,4 glycosidic cleavage linkages in human.
Reference: Bookbinder LH, Hofer A, Haller MF, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release,2006;114(2):230-241
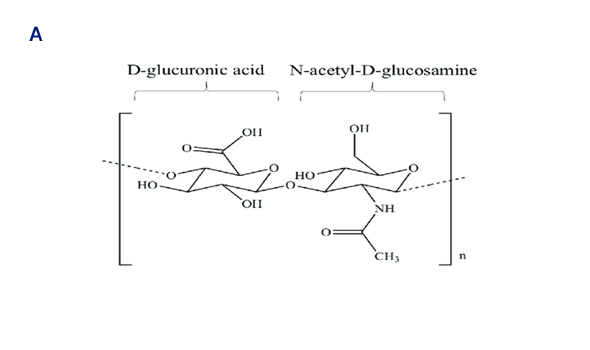
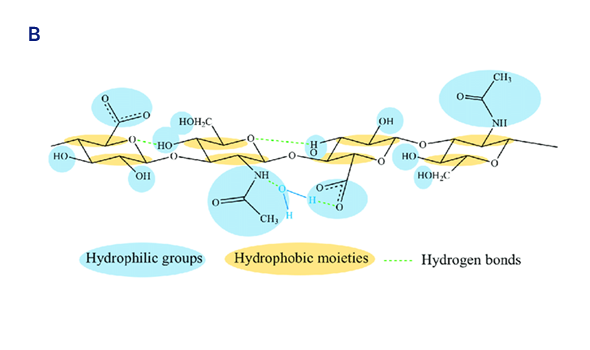
Chemical structures of HA disaccharide unit (A) and HA tetra saccharide unit where the hydrophilic functional groups and the hydrophobic moieties are respectively evidenced in blue and yellow, while the hydrogen bonds are represented by green dashed lines (B).
Hyaluronic Acid in the Third Millennium: Polymers 10,701 MDPI June 2018 Arianna Fallacara ID , Erika Baldini, Stefano Manfredini * and Silvia Vertuani
Transforming Biologics Sub-Q Delivery Paradigm to meet the significant needs of patients
 is a highly purified DIFFerentiated rHuPH20 (recombinant human hyaluronidase PH20) with Huonslab process patent for manufacturing methods of the rHuPH20 in its full length of 447 amino acids.
is a highly purified DIFFerentiated rHuPH20 (recombinant human hyaluronidase PH20) with Huonslab process patent for manufacturing methods of the rHuPH20 in its full length of 447 amino acids.
rHuPH20 has approx. 100 times greater purity than the animal testes derived hyaluronidases and HYLENEX®(Halozyme, US) is the only FDA approved recombinant human hyaluronidase(150 USP units/mL/vial) since 2005.
In the high dose of more than 10 000 IU, rHuPH20 is co-formulated with a partner antibody in a single dose vial, mostly in oncology, drastically reducing patient’s severe burden of hours long IV infusion time to SC injection in just 2-8 minutes.
 platform is a highly acclaimed and Huonslab proprietary hyaluronidase-based drug delivery technology, enabling the conversion of biologics IV infusions to Sub-Q delivery.
platform is a highly acclaimed and Huonslab proprietary hyaluronidase-based drug delivery technology, enabling the conversion of biologics IV infusions to Sub-Q delivery.
The platform is applied for both, as API for co-formulations with antibodies, and as Drug Product, HLB3-002 that is being developed at IND stage in S. Korea.
Recombinant human hyaluronidase as Drug Substance is an API in the US and non-medical component, an excipient in Europe and in S. Korea, currently.
 (rHuPH20)
(rHuPH20)
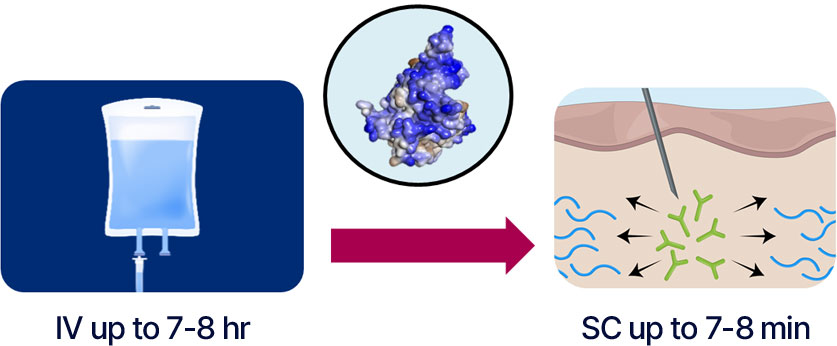
rHuPH20 degrades hyaluronan in the SC space, increasing tissue permeability and facilitating rapid, Large-Volume Sub-Q Delivery of co-administered drugs. The enzymatic reaction is reversible, transient, and Non-inflammatory.
Patient's burden of hours-long infusion time can be immensely reduced with only 5 to 7 minutes SC injection of rHuPH20 in co-administration of partner biologics.
When administered as SC injection with a partner antibody,  being rHuPH20 is anticipated to temporarily depolymerize HA in the hypodermis, resulting in the reduction of Hyaluronan viscosity and the increased tissue permeability and bulk fluid flow.
being rHuPH20 is anticipated to temporarily depolymerize HA in the hypodermis, resulting in the reduction of Hyaluronan viscosity and the increased tissue permeability and bulk fluid flow.
rHuPH20 enables the conversion of High-Volume, High-Dose Biologics IV infusion to SC injection with volume of 5-15mL based on the currently marketed co-formulations, mostly with solid market performance in Oncology.
HLB3-002
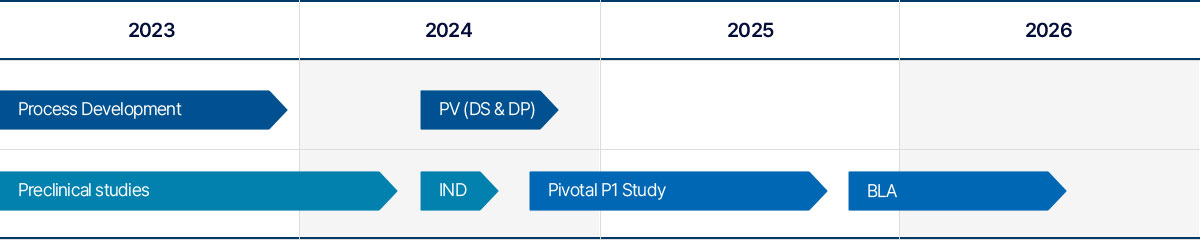
HLB3-002 is being developed as albumin-free and preservative-free recombinant human PH20 drug product in 1500 IU/mL/vial in S. Korea.
We are planning to complete pivotal P1 study by Q3 2025 followed by MFDS BLA approval in 2026.
As of August 2024
Market segmentation of the animal testes-derived and recombinant human hyaluronidases in the clinical practice is as classified and described below.
US Approved Indications
- Subcutaneous fluid
infusion(hypodermoclysis) - As an adjuvant to accelerate the absorption and dispersion of drugs in subcutaneous tissue or to manage extravasation, and
- as an adjunct to promote the absorption of contrast media in urinary tract angiography(subcutaneous urography)
Plastic Surgery
- Filler over-correction/Lumpy filler
- Tyndall effect
- Filler asymmetry
- Concerns for vascular occlusion
- Inflammatory or infectious nodule
Reference: Hyaluronidase for Treating Complications Related to HA Fillers: A National Plastic Surgeon Survey
Emergency Care Unit
- Reducing swelling from insect bites, burns, and injuries
- Treating post-surgical edema and hematoma (Chest and breast surgery)
Ophthalmology & Anesthesiology
- Enhancing anesthetic effect for eye surgeries (retrobulbar, peribulbar, sub-Tenon's block).
- Reducing pain from local anesthetics SC injection
- Facilitating rapid penetration of local anesthetics and preventing prolonged anesthesia.
Global hyaluronidase market reached USD 909.5 million in 2022 and anticipated to grow at a CAGR of 8.4% from 2023 to 2030.
North America presented the largest revenue share of 38.0% and Asia Pacific is anticipated to grow at the fastest CAGR of 9.3% during the forecast period from 2023 to 2030 (Source: Grand View Research, US www.grandviewresearch.com)

